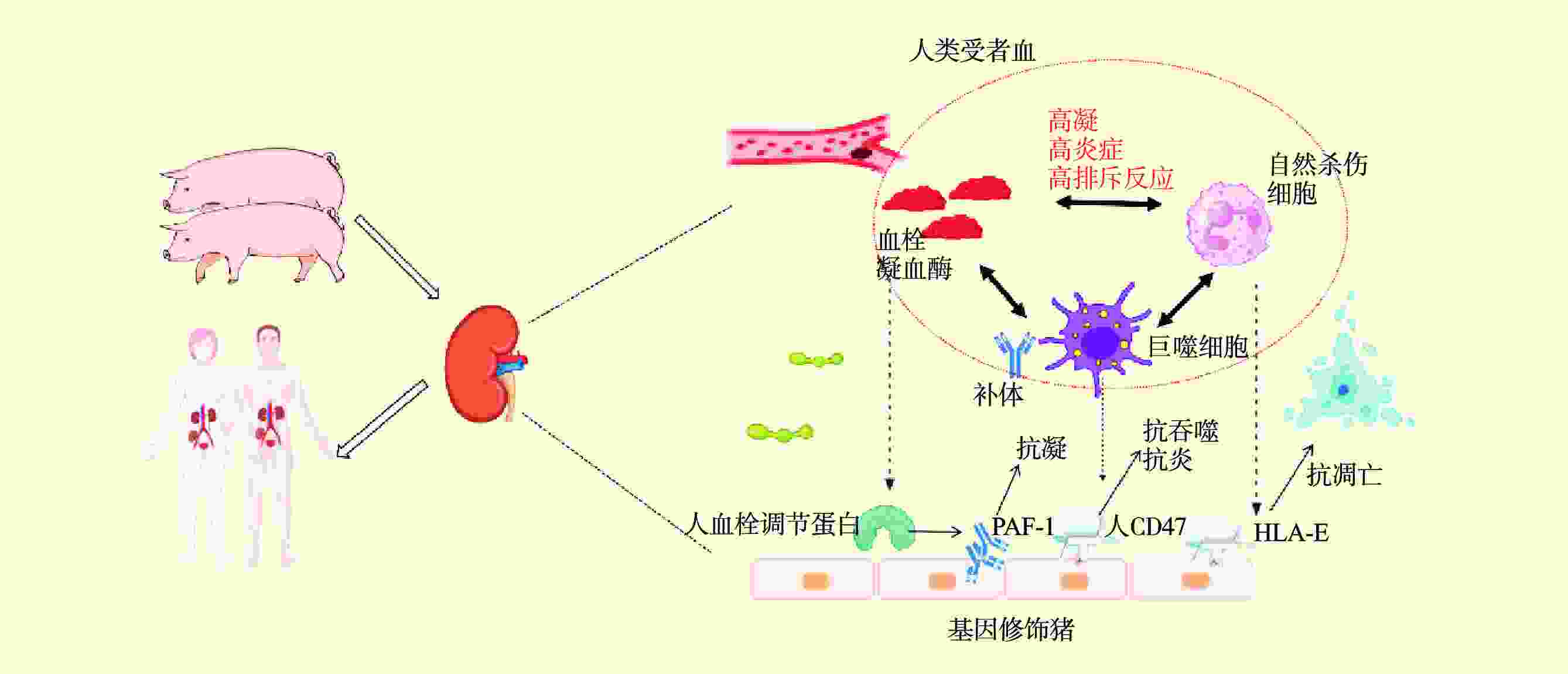
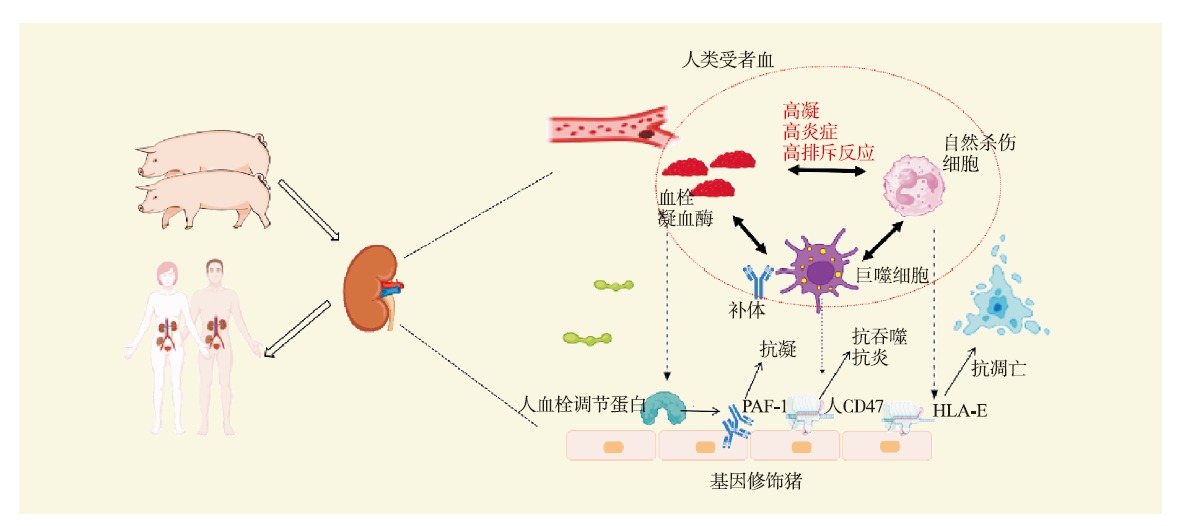
| [1] |
WANG X, MO Y, YUAN Y, et al. Exploring the influencing factors of unmet palliative care needs in Chinese patients with end-stage renal disease undergoing maintenance hemodialysis: a cross-sectional study[J]. BMC Palliat Care, 2023, 22(1): 113. DOI: 10.1186/s12904-023-01237-x.
|
| [2] |
LENTINE KL, SMITH JM, HART A, et al. OPTN/SRTR 2020 annual data report: kidney[J]. Am J Transplant, 2022, 22(Suppl 2): 21-136. DOI: 10.1111/ajt.16982.
|
| [3] |
HUANG J. Expert consensus on clinical trials of human xenotransplantation in China[J]. Health Care Sci, 2022, 1: 7-10. DOI: 10.1002/hcs2.6.
|
| [4] |
PEREIRA-MARTINS DA, WEINHÄUSER I, COELHO-SILVA JL, et al. MLL5 improves ATRA driven differentiation and promotes xenotransplant engraftment in acute promyelocytic leukemia model[J]. Cell Death Dis, 2021, 12(4): 371. DOI: 10.1038/s41419-021-03604-z.
|
| [5] |
COOPER DKC, GASTON R, ECKHOFF D, et al. Xenotransplantation-the current status and prospects[J]. Br Med Bull, 2018, 125(1): 5-14. DOI: 10.1093/bmb/ldx043.
|
| [6] |
LAI L, KOLBER-SIMONDS D, PARK KW, et al. Production of alpha-1, 3-galactosyltransferase knockout pigs by nuclear transfer cloning[J]. Science, 2002, 295(5557): 1089-1092. DOI: 10.1126/science.1068228.
|
| [7] |
SYKES M, SACHS DH. Progress in xenotransplantation: overcoming immune barriers[J]. Nat Rev Nephrol, 2022, 18(12): 745-761. DOI: 10.1038/s41581-022-00624-6.
|
| [8] |
MOAZAMI N, STERN JM, KHALIL K, et al. Pig-to-human heart xenotransplantation in two recently deceased human recipients[J]. Nat Med, 2023, 29(8): 1989-1997. DOI: 10.1038/s41591-023-02471-9.
|
| [9] |
ZHANG X, WANG Q, ZHAO J, et al. The resurgent landscape of xenotransplantation of pig organs in nonhuman primates[J]. Sci China Life Sci, 2021, 64(5): 697-708. DOI: 10.1007/s11427-019-1806-2.
|
| [10] |
ARABI TZ, SABBAH BN, LERMAN A, et al. Xenotransplantation: current challenges and emerging solutions[J]. Cell Transplant, 2023, 32: 9636897221148771. DOI: 10.1177/09636897221148771.
|
| [11] |
LÄNGIN M, MAYR T, REICHART B, et al. Consistent success in life-supporting porcine cardiac xenotransplantation[J]. Nature, 2018, 564(7736): 430-433. DOI: 10.1038/s41586-018-0765-z.
|
| [12] |
KIM SC, MATHEWS DV, BREEDEN CP, et al. Long-term survival of pig-to-rhesus macaque renal xenografts is dependent on CD4 T cell depletion[J]. Am J Transplant, 2019, 19(8): 2174-2185. DOI: 10.1111/ajt.15329.
|
| [13] |
GRIFFITH BP, GOERLICH CE, SINGH AK, et al. Genetically modified porcine-to-human cardiac xenotransplantation[J]. N Engl J Med, 2022, 387(1): 35-44. DOI: 10.1056/NEJMoa2201422.
|
| [14] |
LOCKE JE, KUMAR V, ANDERSON D, et al. Normal graft function after pig-to-human kidney xenotransplant[J]. JAMA Surg, 2023, 158(10): 1106-1108. DOI: 10.1001/jamasurg.2023.2774.
|
| [15] |
LI J, XU Y, ZHANG J, et al. Single-cell transcriptomic analysis reveals transcriptional and cell subpopulation differences between human and pig immune cells[J]. Genes Genomics, 2024, 46(3): 303-322. DOI: 10.1007/s13258-023-01456-9.
|
| [16] |
MATSUMOTO S, ABALOVICH A, WYNYARD S, et al. Patients' opinions 10 years after receiving encapsulated porcine islet xenotransplantation without immunosuppression[J]. Xenotransplantation, 2023, 30(3): e12798. DOI: 10.1111/xen.12798.
|
| [17] |
王维, 莫朝辉, 叶斌, 等. 新生猪胰岛移植治疗糖尿病病人的临床研究[J]. 中南大学学报(医学版), 2011, 36(12): 1134-1140. DOI: 10.3969/j.issn.1672-7347.2011.12.002.WANG W, MO ZH, YE B, et al. A clinical trial of xenotransplantation of neonatal pig islets for diabetic patients[J]. J Cent South Univ (Med Sci), 2011, 36(12): 1134-1140. DOI: 10.3969/j.issn.1672-7347.2011.12.002.
|
| [18] |
张玄, 王琳, 张洪涛, 等. 多基因编辑猪-猴心脏、肝脏、肾脏移植临床前研究初步报道[J]. 器官移植, 2021, 12(1): 51-56. DOI: 10.3969/j.issn.1674-7445.2021.01.008.ZHANG X, WANG L, ZHANG HT, et al. Preliminary report of preclinical trial of multi-genome engineering pig-to-macaque heart, liver and kidney transplantation[J]. Organ Transplant, 2021, 12(1): 51-56. DOI: 10.3969/j.issn.1674-7445.2021.01.008.
|
| [19] |
FENG H, LI T, DU J, et al. Both natural and induced anti-sda antibodies play important roles in GTKO pig-to-rhesus monkey xenotransplantation[J]. Front Immunol, 2022, 13: 849711. DOI: 10.3389/fimmu.2022.849711.
|
| [20] |
杨树军, 卫浩, 许勇, 等. 六基因编辑猪-食蟹猴异种肾移植围手术期监测初步报道[J]. 器官移植, 2023, 14(4): 521-528. DOI: 10.3969/j.issn.1674-7445.2023.04.008.YANG SJ, WEI H, XU Y, et al. Preliminary report of perioperative monitoring of six-gene-edited pig-to-cynomolgus monkey kidney xenotransplantation[J]. Organ Transplant, 2023, 14(4): 521-528. DOI: 10.3969/j.issn.1674-7445.2023.04.008.
|
| [21] |
高菲, 王煜, 杜嘉祥, 等. 遗传修饰猪模型在生物医学及农业领域研究进展及应用[J]. 遗传, 2023, 45(1): 6-28. DOI: 10.16288/j.yczz.22-313.GAO F, WANG Y, DU JX, et al. Advances and applications of genetically modified pig models in biomedical and agricultural field[J]. Hereditas, 2023, 45(1): 6-28. DOI: 10.16288/j.yczz.22-313.
|
| [22] |
淮国丽, 杜嘉祥, 潘登科. 基因编辑猪用于急性肝衰竭治疗的路径探讨[J]. 临床肝胆病杂志, 2022, 38(10): 2214-2218. DOI: 10.3969/j.issn.1001-5256.2022.10.004.HUAI GL, DU JX, PAN DK. The discussion on the genetically modified pigs for the treatment of acute liver failure[J]. J Clin Hepatol, 2022, 38(10): 2214-2218. DOI: 10.3969/j.issn.1001-5256.2022.10.004.
|
| [23] |
HARISA GI, FARIS TM, SHERIF AY, et al. Gene-editing technology, from macromolecule therapeutics to organ transplantation: applications, limitations, and prospective uses[J]. Int J Biol Macromol, 2023, 253(Pt 5): 127055. DOI: 10.1016/j.ijbiomac.2023.127055.
|
| [24] |
GANCHIKU Y, RIELLA LV. Pig-to-human kidney transplantation using brain-dead donors as recipients: one giant leap, or only one small step for transplantkind?[J]. Xenotransplantation, 2022, 29(3): e12748. DOI: 10.1111/xen.12748.
|
| [25] |
DENNER J. Microchimerism, PERV and xenotransplantation[J]. Viruses, 2023, 15(1): 190. DOI: 10.3390/v15010190.
|
| [26] |
NIU D, WEI HJ, LIN L, et al. Inactivation of porcine endogenous retrovirus in pigs using CRISPR-Cas9[J]. Science, 2017, 357(6357): 1303-1307. DOI: 10.1126/science.aan4187.
|
| [27] |
窦科峰, 张玄. 临床异种器官移植十大问题的思考[J]. 器官移植, 2022, 13(4): 411-416. DOI: 10.3969/j.issn.1674-7445.2022.04.001.DOU KF, ZHANG X. Reflection on 10 problems of clinical xenotransplantation[J]. Organ Transplant, 2022, 13(4): 411-416. DOI: 10.3969/j.issn.1674-7445.2022.04.001.
|
| [28] |
MOHIUDDIN MM, SINGH AK, CORCORAN PC, et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO. hCD46. hTBM pig-to-primate cardiac xenograft[J]. Nat Commun, 2016, 7: 11138. DOI: 10.1038/ncomms11138.
|
| [29] |
BIKHET M, IWASE H, YAMAMOTO T, et al. What therapeutic regimen will be optimal for initial clinical trials of pig organ transplantation?[J]. Transplantation, 2021, 105(6): 1143-1155. DOI: 10.1097/TP.0000000000003622.
|
| [30] |
SÖLLNER JH, METTENLEITER TC, PETERSEN B. Genome editing strategies to protect livestock from viral infections[J]. Viruses, 2021, 13(10): 1996. DOI: 10.3390/v13101996.
|
| [31] |
LOVELAND BE, MILLAND J, KYRIAKOU P, et al. Characterization of a CD46 transgenic pig and protection of transgenic kidneys against hyperacute rejection in non-immunosuppressed baboons[J]. Xenotransplantation, 2004, 11(2): 171-183. DOI: 10.1046/j.1399-3089.2003.00103.x.
|
| [32] |
CHABAN R, COOPER DKC, PIERSON RN 3RD. Pig heart and lung xenotransplantation: present status[J]. J Heart Lung Transplant, 2022, 41(8): 1014-1022. DOI: 10.1016/j.healun.2022.04.010.
|
| [33] |
DELAURA I, ANWAR IJ, LADOWSKI J, et al. Attitudes of patients with renal disease on xenotransplantation: a systematic review[J]. Xenotransplantation, 2023, 30(2): e12794. DOI: 10.1111/xen.12794.
|
| [34] |
COOPER DKC, HARA H, IWASE H, et al. Justification of specific genetic modifications in pigs for clinical organ xenotransplantation[J]. Xenotransplantation, 2019, 26(4): e12516. DOI: 10.1111/xen.12516.
|
| [35] |
DENNER J. The origin of porcine endogenous retroviruses (PERVs)[J]. Arch Virol, 2021, 166(4): 1007-1013. DOI: 10.1007/s00705-020-04925-8.
|
| [36] |
WAHID B, USMAN S, ALI A, et al. Therapeutic strategies of clustered regularly interspaced palindromic repeats-cas systems for different viral infections[J]. Viral Immunol, 2017, 30(8): 552-559. DOI: 10.1089/vim.2017.0055.
|
| [37] |
LIU Y, QIN L, TONG R, et al. Regulatory changes in China on xenotransplantation and related products[J]. Xenotransplantation, 2020, 27(3): e12601. DOI: 10.1111/xen.12601.
|
| [38] |
COOPER DKC, HARA H, IWASE H, et al. Clinical pig kidney xenotransplantation: how close are we?[J]. J Am Soc Nephrol, 2020, 31(1): 12-21. DOI: 10.1681/ASN.2019070651.
|
| [39] |
FISHMAN JA. Prevention of infection in xenotransplantation: designated pathogen-free swine in the safety equation[J]. Xenotransplantation, 2020, 27(3): e12595. DOI: 10.1111/xen.12595.
|
| [40] |
YAMAMOTO T, IWASE H, PATEL D, et al. Old world monkeys are less than ideal transplantation models for testing pig organs lacking three carbohydrate antigens (triple-knockout)[J]. Sci Rep, 2020, 10(1): 9771. DOI: 10.1038/s41598-020-66311-3.
|
| [41] |
MOHIUDDIN MM, SINGH AK, SCOBIE L, et al. Graft dysfunction in compassionate use of genetically engineered pig-to-human cardiac xenotransplantation: a case report[J]. Lancet, 2023, 402(10399): 397-410. DOI: 10.1016/S0140-6736(23)00775-4.
|
| [42] |
PULLEN LC. Xenotransplant: coming soon?[J]. Am J Transplant, 2022, 22(4): 1003-1004. DOI: 10.1111/ajt.16651.
|
| [43] |
TECTOR AJ, ADAMS AB, TECTOR M. Current status of renal xenotransplantation and next steps[J]. Kidney360, 2023, 4(2): 278-284. DOI: 10.34067/KID.0007152021.
|




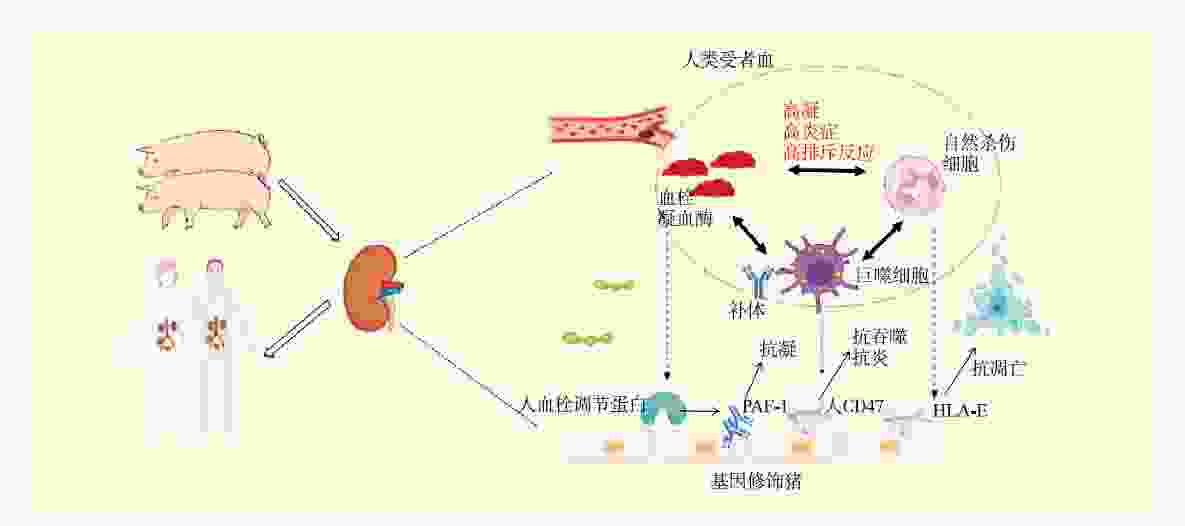
 下载:
下载: